Interventional Cardiology
INSPIRON® is a 3rd generation Drug Eluting Stent designed to create a fast and homogeneous endothelialization. Its platform is the CRONUS NE® Stent, a stent which design, material (CoCr), thin struts (75 μm) and delivery system gives it good navigability, flexibility, good crossover profile, moderate radiopacity and high balloon rupture pressure. Its abluminal coating is composed of a mixture of PLA and PLGA polymers and a low Sirolimus dosage resulting in a moderate drug elution profile.
Main Characteristics
Advanced design with thin struts
- Cobalt chromium alloy.
- 75μm strut thickness.
- Excellent radial force.

Rounded structure to avoid artery injury.
“S” connectors for high flexibility and navigability.
Abluminal Coating

Coating thickness – 5μm
100% Biodegradable Polymer

Complete degradation of polymers in CO2 and H2O.
Clinical Trials
Preclinical and Clinical Trial have proven the efficacy and safety of the INSPIRON® Drug-Eluting Stent.
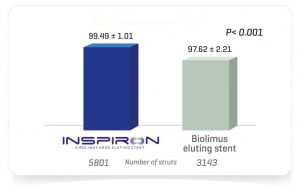
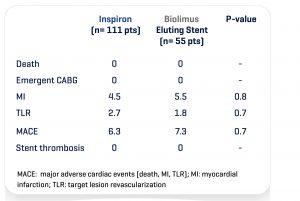
The Destiny Trial, a multicenter randomized study comparing INSPIRON® to a Biolimus-eluting stent, in which 170 patients -194 lesions; demonstrated noninferiority for in stent late lumen loss with p < 0.001 (primary endpoint) and the safety of the stent with no death, no thrombosis and low major adverse cardiac and cerebral events (3.6% vs. 5.45% of Biolimus-eluting stent, p= 0.7) at 9 months. The analysis of 8944 struts by OCT (Optical Coherence Tomography) showed a higher coverage rate at 9 months (99.49% vs. 97.62% for the Biolimus-eluting stent with p value<0.001).
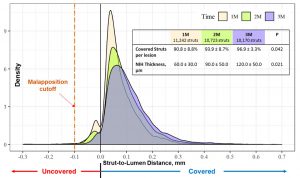
The high rate of struts coverage was also confirmed by the REPAIR study with 97% of the struts covered at 3 months.
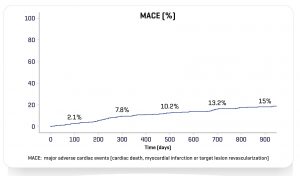
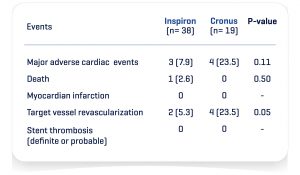
At 300 days, the Real Life Study I (470 patients treated / 799 stents implanted) showed that even among a very complex population (51.3% of diabetics, 68.5% of multiarterials, 58.5% had previously PCI or bypass surgery, 38.9% with bifurcation, 61.9% with type C lesion) INSPIRON®️ demonstrated an excellent efficacy and safety profile with 1.7% cardiac mortality, 5.7% target vessel revascularization and zero possible or definite thrombosis.
DESTINY Trial ¹ ²
Study Design
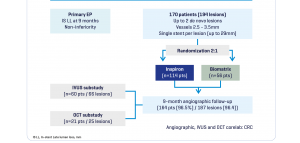
Inspiron Real Life Study I – Novel DES in high-risk patients³
Study Design
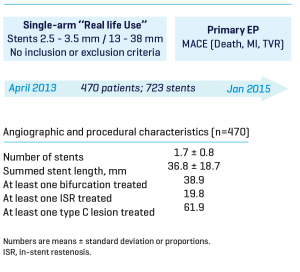
Baseline characteristics
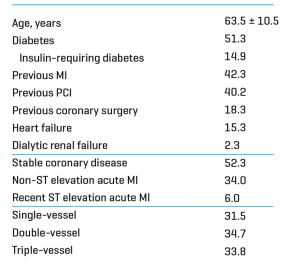
Main Clinical Results
1 – Angiographically Non-inferiority vs. biolimus eluting stent at 9 months.¹
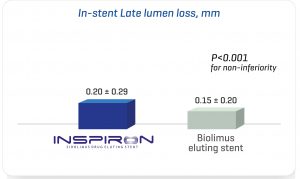
2 – OCT substudies demonstrate that INSPIRON has excellent strut coverage even at 3 months and higher strut coverage compared to Biolimus Eluting Stent with p<0.001 at 9 months.
%Covered Struts – OCT ²
Assessment of Stent Healing6
3 – Proven Safety and Efficacy with low rates of thrombosis in the long-term.
Inspiron Real Life I – Novel DES in high-risk patients³ – 900 days of median follow-up (n=470)*
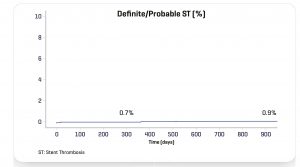
DESTINY Trial
Clinical adverse events (up to 270 days)
First In Human (4 years)
References
- Metallic Limus-eluting stents abluminally coated with biodegradable polymers: angiographic and clinical comparison of a novel ultra-thin Sirolimus Stent versus Biolimus stent in the DESTINY Randomized Trial. Cardiovascular Therapeutics, 2015; 33(6): 367-371.
- Intravascular imaging comparison of two metallic limus-eluting stents abluminally coated with biodegradable polymers: IVUS and OCT results of the DESTINY trial. Int J Cardiovasc Imaging. 2017 Feb;33(2):161-168.
- Clinical performance of a novel ultrathin strut, low-dose, sirolimus-eluting stent with abluminal-only biodegradable polymeric coating for patients undergoing percutaneous coronary intervention in the daily practice. Cardiovasc Diagn Ther. 2015 Dec;5(6):414-9.
- Four-year clinical follow-up of the first-in-man randomized comparison of a novel sirolimus eluting stent with abluminal biodegradable polymer and ultra-thin strut cobalt-chromium alloy: the INSPIRON-I trial. Cardiovasc Diagn Ther 2015;5(4):264-270.
- First-in-man randomized comparison of a novel sirolimus-elutig stent with abluminal biodegradable polymer and thin-strut cobalt-chromium alloy: INSPIRON-I trial. EuroIntervention. 2014;9: 1380-4.
- Multicenter, prospective, randomized study to evaluate by OCT the healing score after stent implantation at 1, 2 and 3 months (NCT03269461).
- Prospective, non-randomized, unicentric, to assess clinical outcomes of the Inspiron Sirolimus Eluting Stent.
| Study | #Patients | Study Design |
| Inspiron I Study | 60 | Multicenter, prospective, randomized study to evaluate safety and efficacy of Inspiron Sirolimus Eluting Stent (NCT01093391) |
| Destiny Trial | 170 | Multicenter, prospective, randomized study to show non-inferiority against Biolimus Eluting Stent (NCT01856088) |
| Inspiron Real Life Registry | 470 | Prospective, non-randomized study of complex population |
| Inspiron Real Life II Registry | 5000 | Multicenter, prospective, non-randomized real-world Registry (NCT03263260) |
| Inspiron Repair | 60 | Multicenter, prospective, randomized study to evaluate by OCT the healing score after stent implantation at 1, 2 and 3 months (NCT03269461) |
| Inspiron Latitude | 500 | Multicenter, prospective, non-randomized real-world study (NCT03471234) |
| Inspiron All-commers | 790 | Prospective, non-randomized, unicentric, to assess clinical outcomes of the Inspiron Sirolimus Eluting Stent. |
Ordering Information
Length
| Diameter | 9mm* | 13mm | 16mm | 19mm | 23mm | 29mm | 33mm | 38mm | 48mm | 58mm |
| 2.25mm | – | 105181 | 105184 | 105186 | 105187 | 105188 | – | – | – | – |
| 2.50mm | 105024* | 105025 | 102633 | 102632 | 105028 | 105029 | 105030 | 104262 | – | – |
| 2.75mm | 105189* | 105190 | 105191 | 105192 | 105193 | 105194 | 105195 | 105196 | – | – |
| 3.00mm | 105031* | 105032 | 102634 | 101335 | 105034 | 105037 | 105038 | 105041 | 113628 | 113632 |
| 3.50mm | 105042* | 105044 | 102635 | 102636 | 105047 | 105048 | 105051 | 105052 | 113629 | 113633 |
| 4.00mm | 105197* | 105198 | 105199 | 110964 | 110965 | 110966 | – | – | – | – |
* Unavailable for CE Market.

