Main Characteristics

Structure & Design
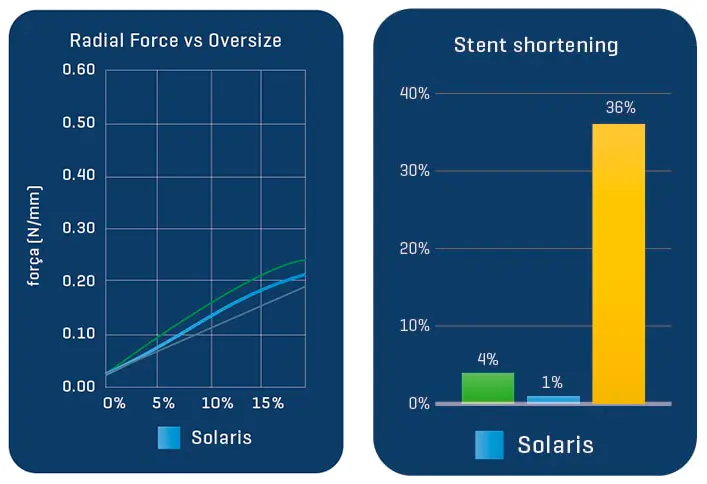
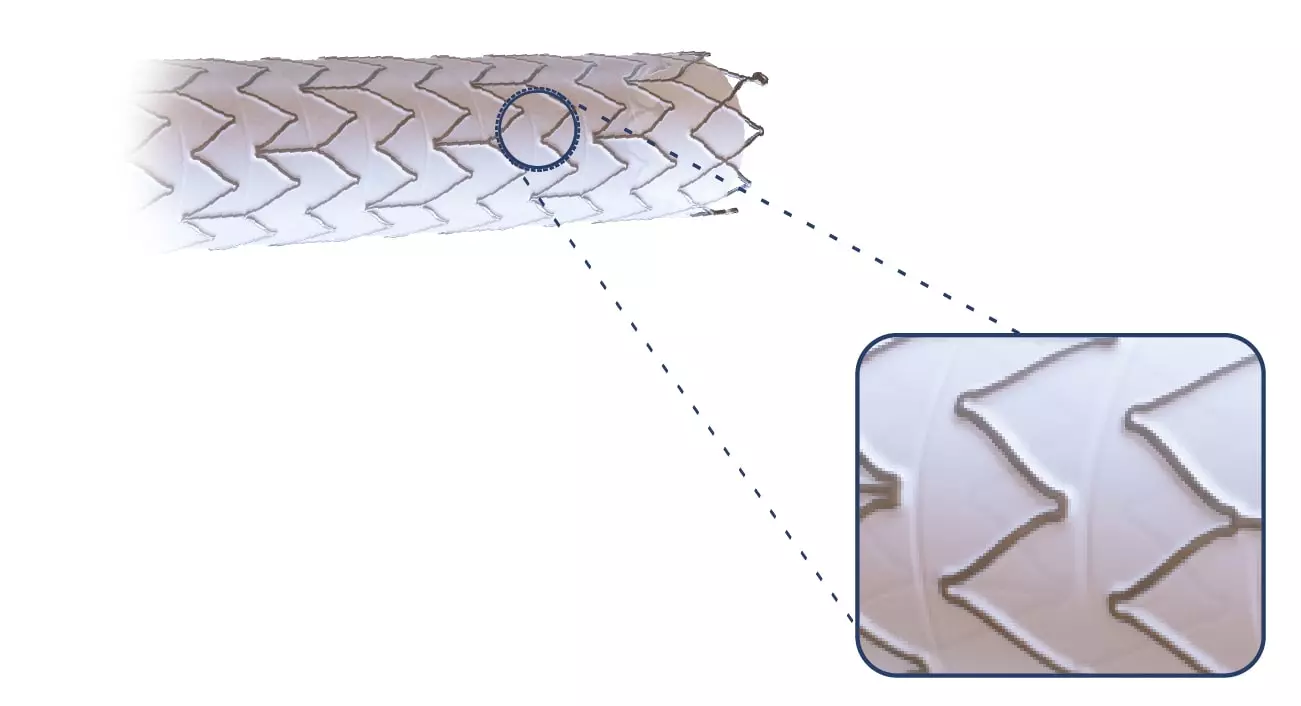
The Solaris Endograft is made from a laser cut of a Nitinol alloy. Due to its design that maintains a distance between one cell and another, the product has high flexibility.
About Nitinol:
Nitinol (from English: Nickel, Titanium, Naval, Ordnance, Laboratory) is a nickel-titanium alloy with high elasticity and memory. In medical materials, thanks to its characteristics, after its delivery of the device the memory effect of the alloy will cause it to tend to return to its original construction design, adapting to the morphology of the artery but providing radial force until its complete expansion.
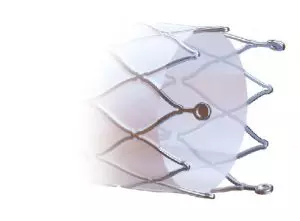
Thinking about not increasing the edge injuries, the Solaris design does not have a “flare” at its ends, and the covering starts at 2mm from the edge.
Also, at the ends, 3 radiopaque tantalum marks were incorporated, which guarantee the product excellent visibility during the procedure.

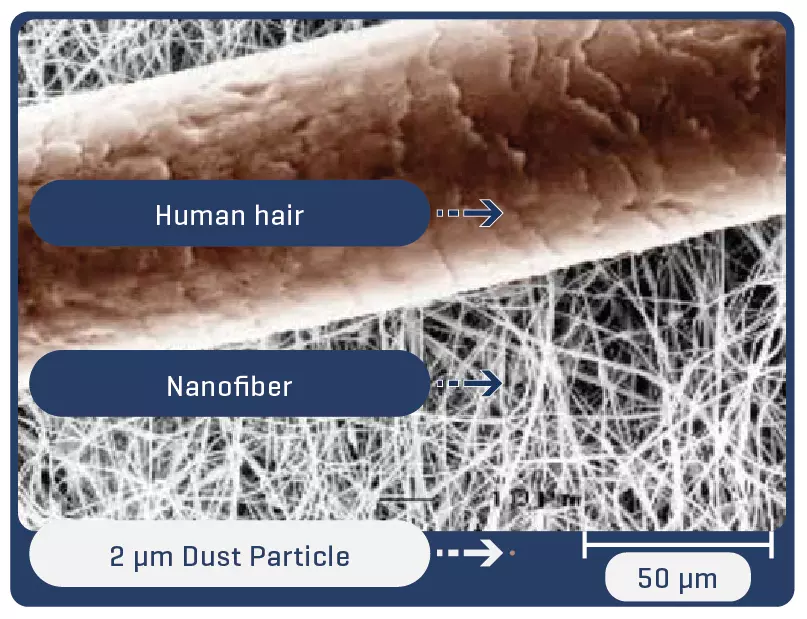
About the electrospinning:
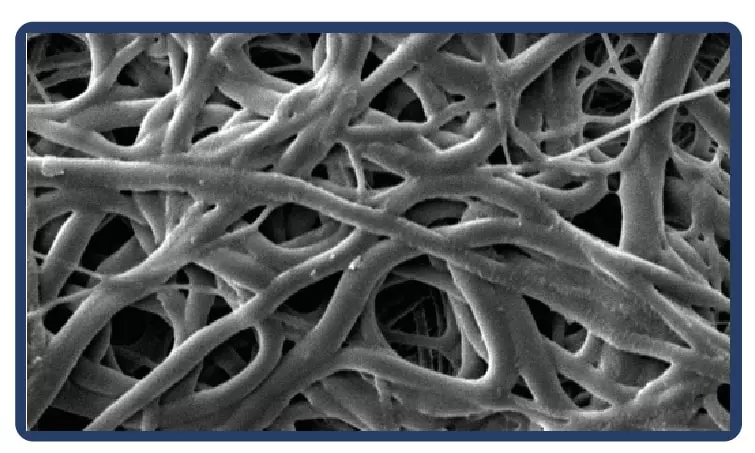
Electrospinning is a technique for producing fibers with diameters on a nanometric scale. Thanks to the process, the fibers are arranged in a multidimensional way, which in the case of PTFE guarantees multidirectional strength and elasticity even in ultrafine fabrics.
Proven in bench tests, the Solaris Endograft membrane has high resilience with the ability to expand up to 3.9x its initial length before rupture by 50% elasticity, which guarantees an important shortening in case of tissue perforation.

Post-puncture area of Solaris fabric with 18g needle (30% of the area of a perforation with the same needle in common ePTFE).
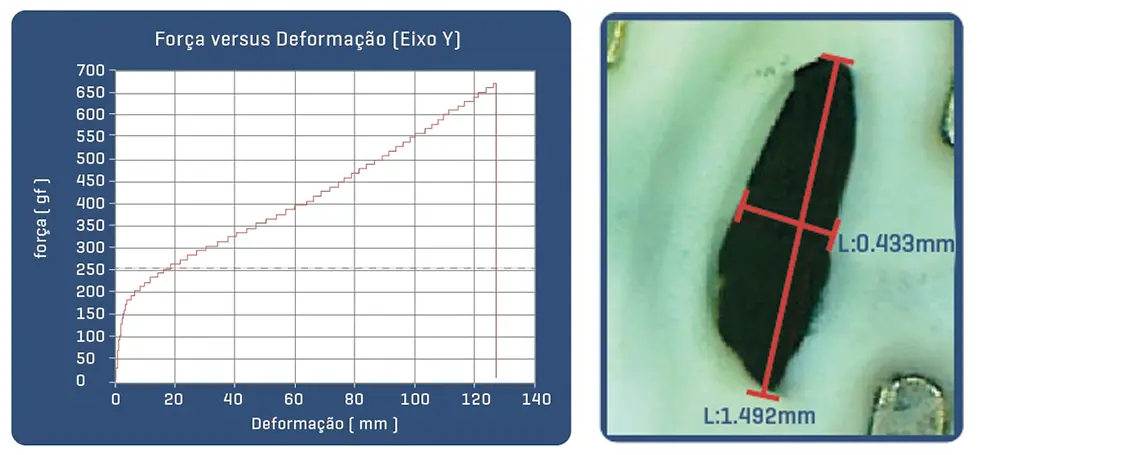
Post-puncture area of common ePTFE fabric with 18g needle.
Delivery System

Clinical Trials
Optical Coherence Tomography and Histopathological Preclinical Evaluation of an Enhanced Polytetrafluoroetylene (PTFE) Covered Stent in a peripheral Swine Animal
Model: Addressing the Anatomical Challenges of the Lower Limb Stenting Procedure
Armando Tellez, Krista N. Dillon, Dane A. Brady, Luciano Curado, Eduardo Cordeiro, Alexander Moreira,
Serge D. Rousselle Alizée Pathology; Thurmont, Maryland; Scitech Medical, São Paulo Brazil
Introduction
Covered stents are widely used to address numerous areas of need in peripheral intervention including degenerated vein grafts, iatrogenic arterial perforations, aneurysm exclusion, iliac artery graft extension, and the chimney/snorkel technique to treat aortic aneurysm. However, PTFE covered stents have a known relative inflexibility which makes deliverability difficult in complex, tortuous, and heavily calcified anatomy. Additionally, current technologies tend to produce kinks resulting in device collapse. This, in turn, can be associated with a high restenosis rate post-implantation, particulary stent-edge restenosis, acute, and subacute thrombosis. In this study we aim to evaluate the performance and vascular response of a new, peripheral covered stent in a large animal model as compared to a commercially available PTFE-covered stent.
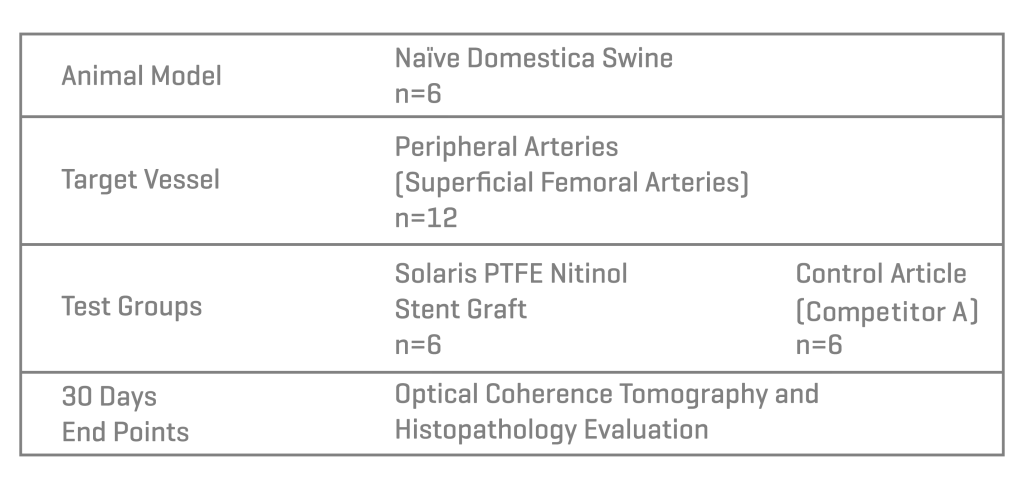
Methods
Six domestic swine were included in this study (45±1kg). Based on angiographic QVA, the superficial femoral arteries were randomized for either the implantation of Solaris (Scitech, São Paulo, Brazil) or Fluency (Bard, Tempe, AZ). Stents utilized were 40mm long for both groups and implanted aiming for a 1.1:1 ratio. Following implantation, animals were recovered and followed for 30 days. At 30 days post-implantation all stents were evaluated under optical coherence tomography (OCT), explanted and subjected to stent integrity analysis and histopathological evaluation.
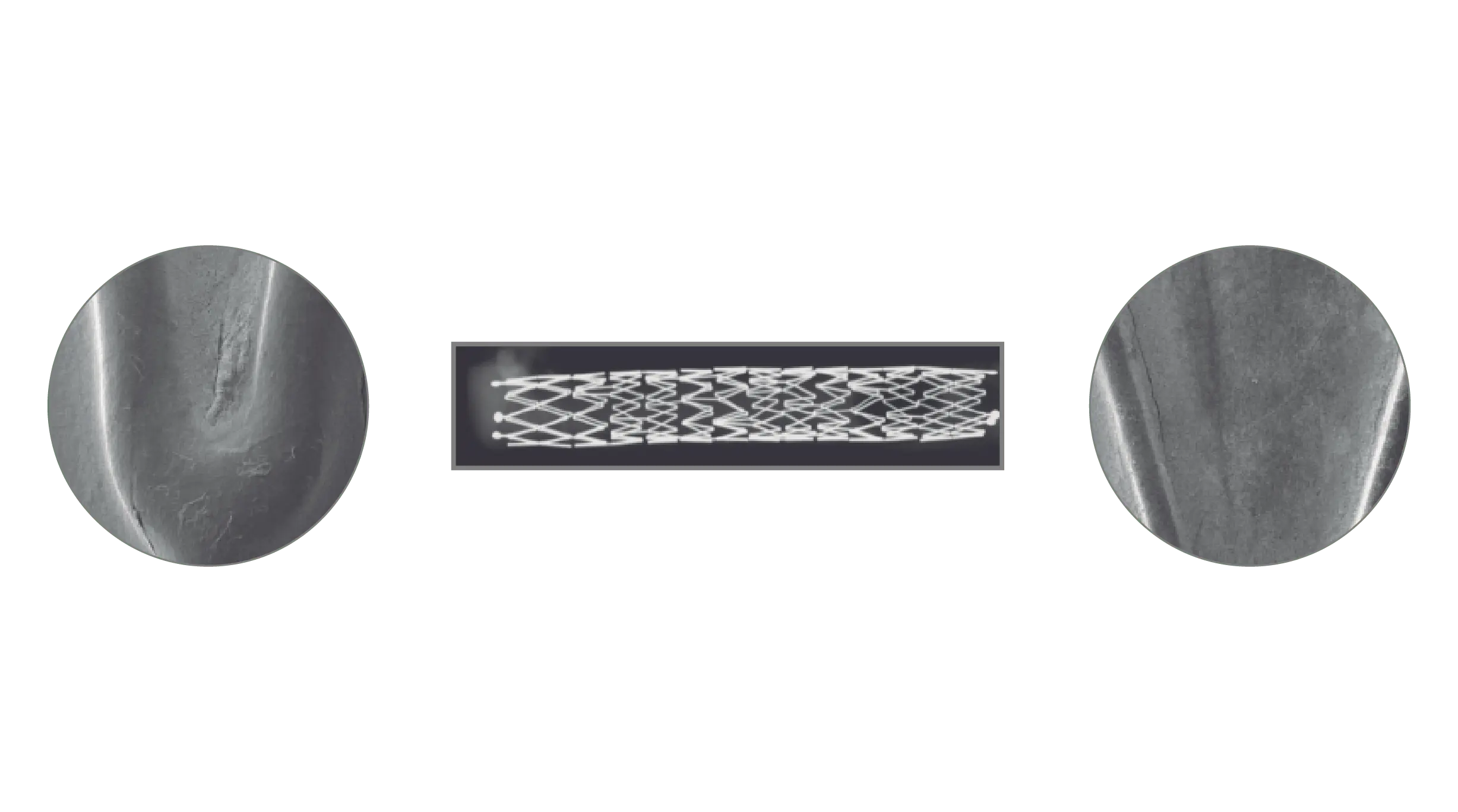
Results
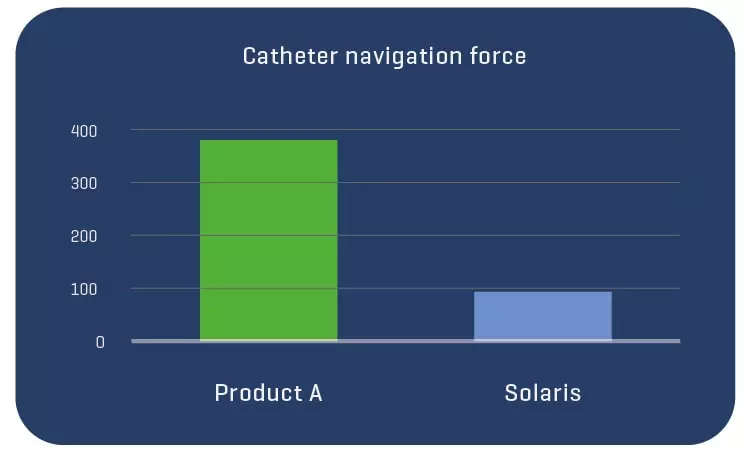
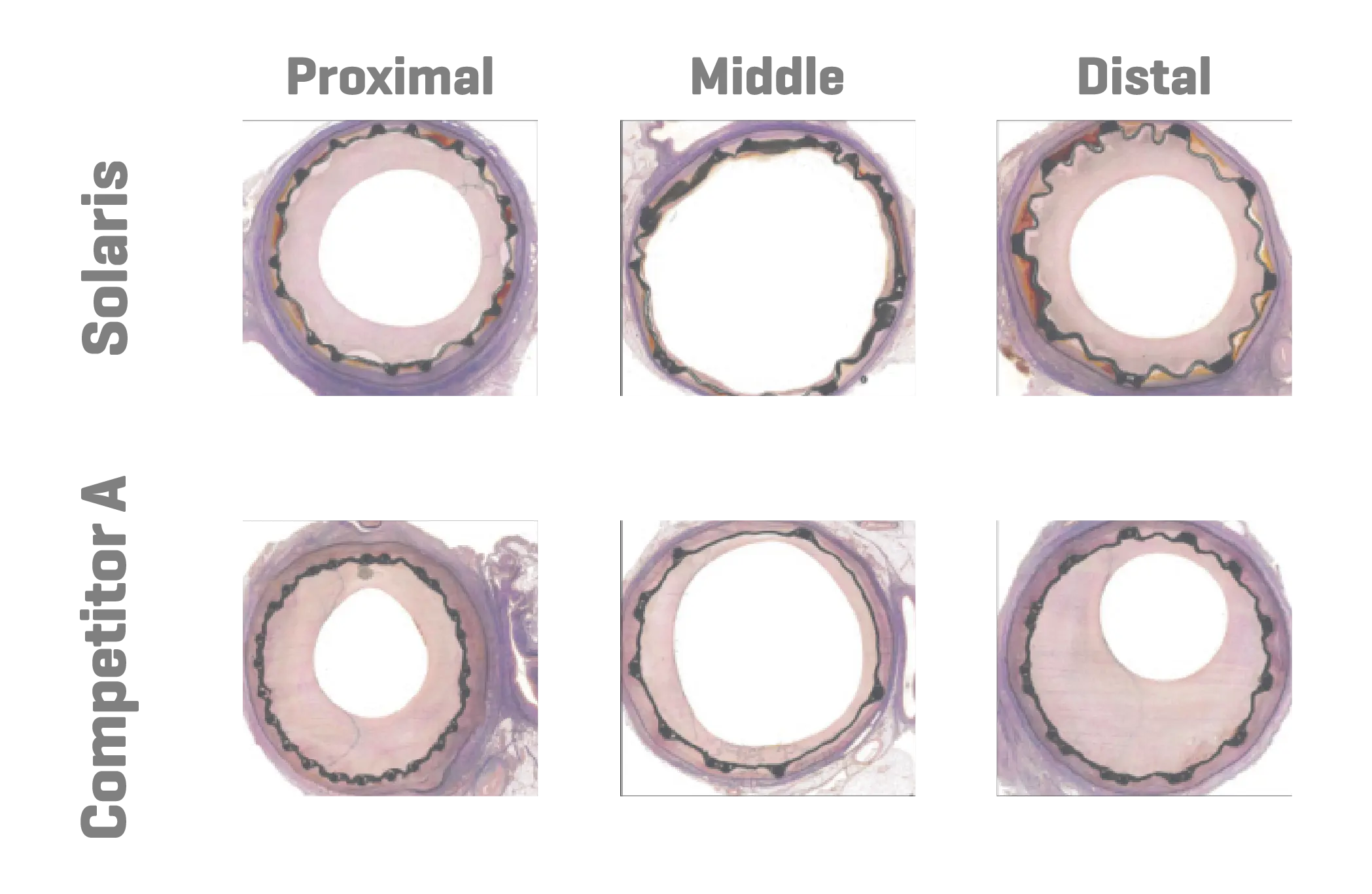
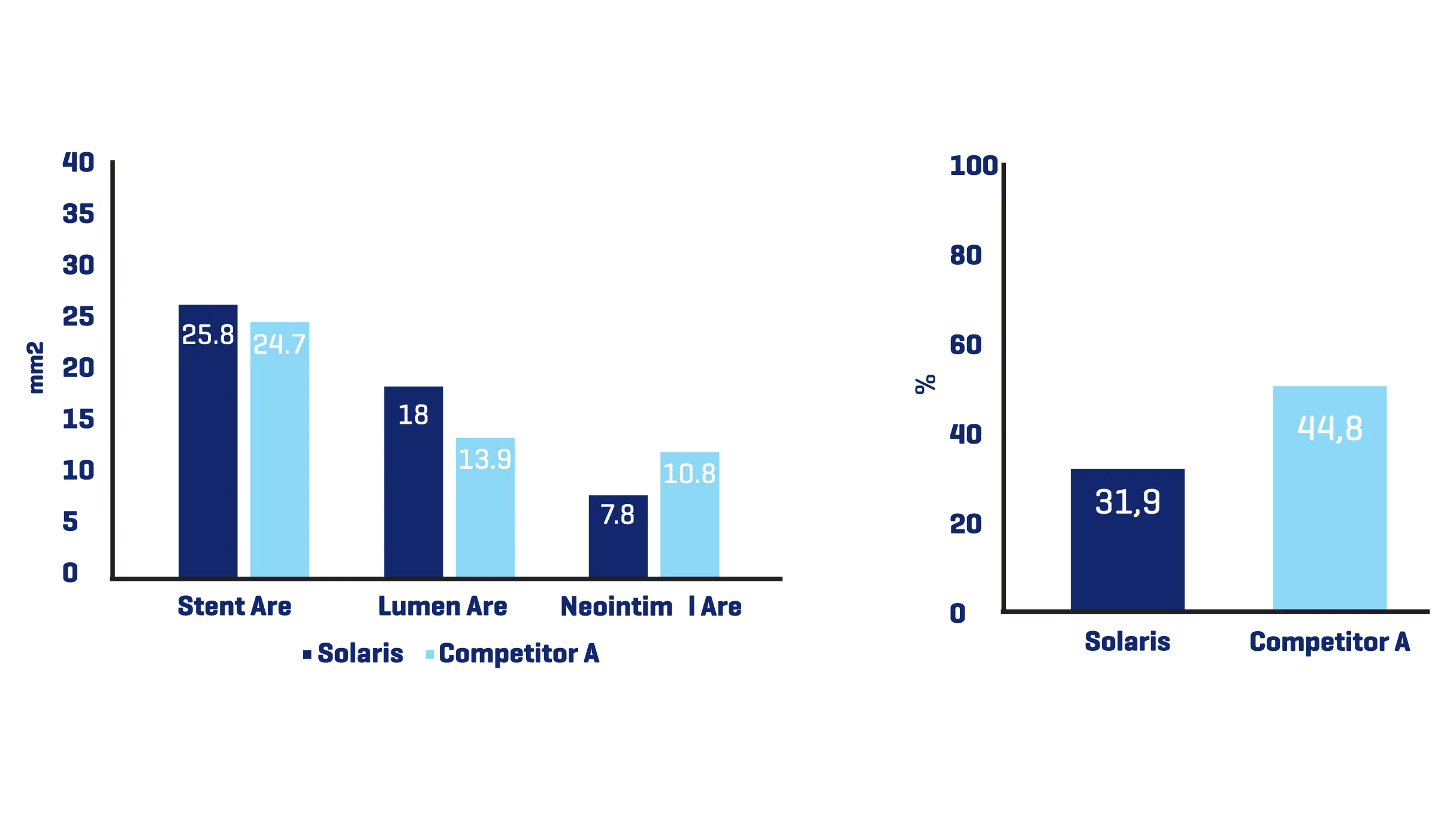
A total of 11 stents were evaluated (Solaris n=6, Fluency n=5), The operator described the Solaris stents as demonstrating a higher navigability when compared to the Fluency stent. The hydrophilic coated delivery system of the Solaris stent allowed a smoother release of the device without any sudden jump and greater geographical precision at implantation. At 30 days, OCT revealed a similar stent area for groups (Solaris 25.8±4,7 vs Fluency 24.7±5mm2) with a lower neointimal area (Solaris 7.8±1.8 vs Fluency 10.8±2.4mm2) compared to control. This led to a higher percentage stenosis in the control group (Solaris 31.9±7 vs Fluency 44.8±6%).
Device description
Low profile (5-7 mm diameter x 40-100 mm in length) self-expandable nitinol peripheral stent. Due to the cell design, the Solaris stent provides superior, ultidirectional flexibility and easy navigability. Three tantalum markers on the extremities allow for precision in delivery.The stent is covered by an ultra-thin, polytetrafluoroethylene (PTFE) membrane. The representative images above illustrate the SEM of the membrane at 170x (left) and 60x (right) objective magnification.
Histopathology showed a slightly lower amount of neointima formation in the Solaris (area percentstenosis ~ 30%) compared to the controls (~37%). There was optimal local biocompatibility in both groups. The Solaris and Fluency stent grafts showed optimal biocompatibility. Both groups showedinstances of increased mural inflammation (in the media and adventitia) primarily due to stent expansion.
Conclusion
The Solaris PTFE-covered peripheral stent demonstrated a resistance to fracture with increased flexibility and navigability and better conformability to artery curvature. The release system allowed for an accurate geographical delivery and the components of the device produced a lower OCT morphometrically – assessed neointimal response when compared to the control group.
Indication for use
CE approved indications
• In the treatment of in-stent restenosis in the venous outflow of hemodialysis patients dialyzing by either an arteriovenous (AV) fistula or AV graft.
• In the treatment of stenosis in the venous outflow of hemodialysis patients dialyzing by an AV graft.
• Restenosis or reocclusion (except vessels on the Central Circulatory System* and Central Nervous System**).
• Dissection (except vessels on the Central Circulatory*System and Central Nervous System**).
• Residual stenosis with impaired perfusion (pressure gradient) following balloon dilatation, especially in stages III and IV according to Fontaine; (except vessels on the Central Circulatory System* and Central Nervous System**).
• Detached arteriosclerotic plaque material and luminal obstruction following balloon dilatation. (except vessels on the Central Circulatory System* and Central Nervous System**).
• Occlusion after thrombolysis or after aspiration and before dilatation. (except vessels on the Central Circulatory System*and Central Nervous System**).*Central Circulatory System* – pulmonary arteries, ascending aorta, aortic arch, descending aorta to aortic bifurcation, coronary arteries, common carotid artery, external carotid artery, internal carotid artery, cerebral arteries, brachycephalic trunk, coronary veins, pulmonary veins, superior vena cava
and inferior vena cava
**Central Nervous System** – the brain, the meninges, and the spinal cord
Ordering Information
CE Mark
Length
| Diameter | 40mm | ø | 60mm | ø | 80mm | ø | 100mm – 9F | ø | 100mm – 8F | ø |
| 5 | 128442 | 8F | 128443 | 8F | 128444 | 8F | – | – | 128445 | 8F |
| 6 | 128446 | 8F | 128447 | 8F | 128448 | 8F | 128438 | 9F | 128941* | 8F |
| 7 | 128449 | 8F | 128450 | 8F | 128451 | 8F | 128439 | 9F | 128942* | 8F |
| 8 | 128943 | 8F | 128944 | 8F | 128945 | 8F | 128440 | 9F | 128946* | 8F |
| 9 | 128099 | 9F | 128100 | 9F | 128101 | 9F | 128441 | 9F | – | – |
| 10 | 129447 | 9F | 129448 | 9F | 129449 | 9F | – | – | – | – |
* Coming Soon
LATAM/ASIA
Length
| Diameter | 40 | ø | 60 | ø | 80 | ø | 100 | ø |
| 5 | 112429 | 8F | 112430 | 8F | 112431 | 8F | 112432 | 8F |
| 6 | 112434 | 8F | 112435 | 8F | 112436 | 8F | 111715 | 9F |
| 7 | 112439 | 8F | 112440 | 8F | 112441 | 8F | 111720 | 9F |
| 8 | 112444 | 8F | 112445 | 8F | 112446 | 8F | 111725 | 9F |
| 9 | 111727 | 9F | 111728 | 9F | 111729 | 9F | 111730 | 9F |