Abstract
Background
The current gold standard of coronary drug-eluting stents (DES) consists of metal alloys with thinner struts and bioresorbable polymers.
Objectives
Our aim was to compare an ultrathin strut, sirolimus-eluting stent (Inspiron®) with other third-generation DES platforms in patients with ST-elevation myocardial infarction (STEMI) submitted to primary percutaneous coronary intervention (PCI).
Methods
We analyzed data from a STEMI multicenter registry from reference centers in the South Region of Brazil. All patients were submitted to primary PCI, either with Inspiron® or other second- or third-generation DES. Propensity score matching (PSM) was computed to generate similar groups (Inspiron® versus other stents) in relation to clinical and procedural characteristics. All hypothesis tests had a two-sided significance level of 0.05.
Results
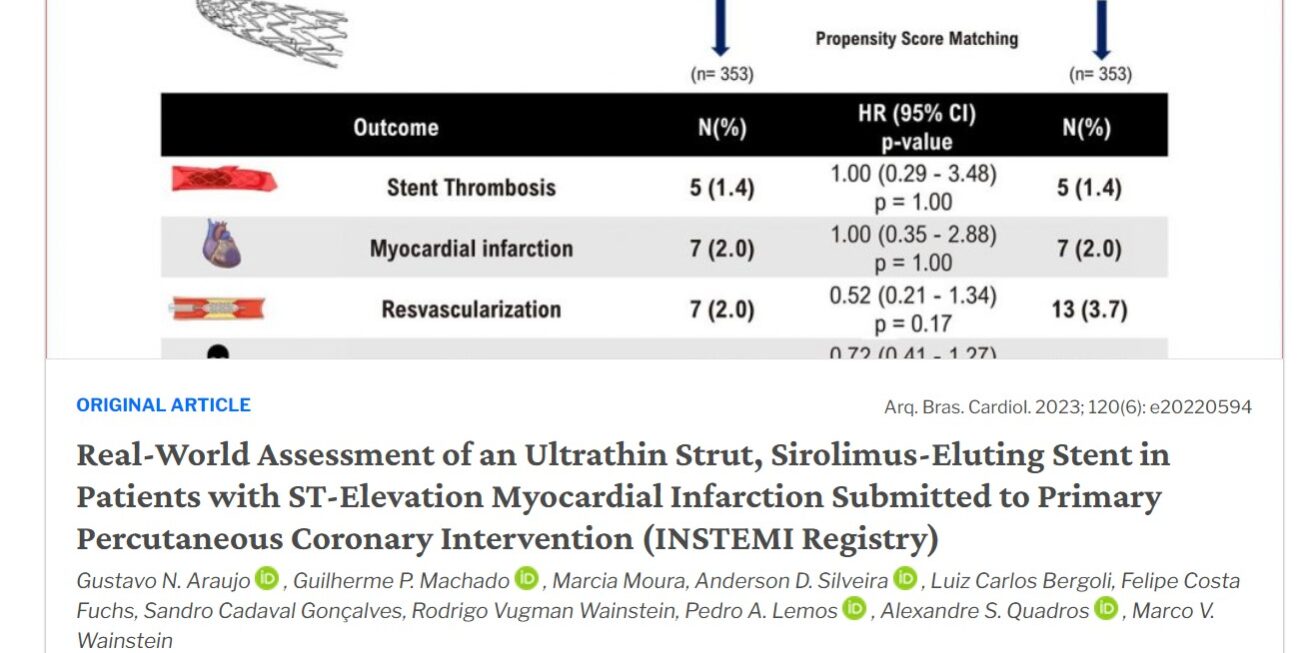
From January 2017 to January 2021, 1711 patients underwent primary PCI, and 1417 patients met our entry criteria (709 patients in the Inspiron® group and 708 patients in the other second- or third-generation DES group). After PSM, the study sample was comprised of 706 patients (353 patients in the Inspiron® group and 353 patients in the other the other second- or third-generation DES group). The rates of target vessel revascularization (OR 0.52, CI 0.21 – 1.34, p = 0.173), stent thrombosis (OR 1.00, CI 0.29 – 3.48, p = 1.000), mortality (HR 0.724, CI 0.41 – 1.27, p = 0.257), and major cardiovascular outcomes (OR 1.170, CI 0.77 – 1.77, p = 0.526) were similar between groups after a median follow-up of 17 months.
Conclusion
Our findings show that Inspiron® was effective and safe when compared to other second- or third-generation DES in a contemporary cohort of real-world STEMI patients submitted to primary PCI.